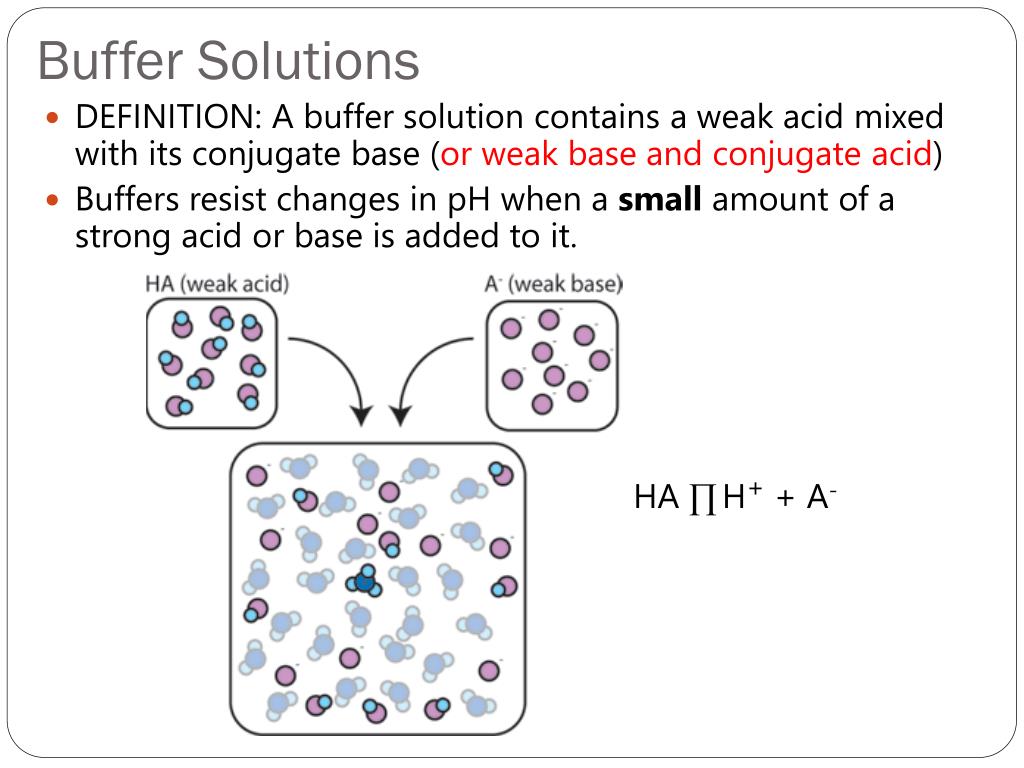
Titration Buffer Solution . A buffer solution is a mixture of a weak acid and its. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. Learn how buffer solutions work, how to. Find out how to determine the. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a buffer solution is a solution that resists ph change on dilution or addition of acid or base. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of.
from www.slideserve.com
A buffer solution is a mixture of a weak acid and its. Find out how to determine the. Learn how buffer solutions work, how to. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. a buffer solution is a solution that resists ph change on dilution or addition of acid or base. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is.
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242
Titration Buffer Solution A buffer solution is a mixture of a weak acid and its. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Learn how buffer solutions work, how to. Find out how to determine the. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. a buffer solution is a solution that resists ph change on dilution or addition of acid or base. A buffer solution is a mixture of a weak acid and its.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube Titration Buffer Solution a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. A buffer solution is a mixture. Titration Buffer Solution.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Buffer Solution Learn how buffer solutions work, how to. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. Find out how to determine the. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region. Titration Buffer Solution.
From www.youtube.com
NCEA L3 Chem Buffers, Buffer Region & Preparation of Buffer Solutions Titration Buffer Solution Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. Learn how buffer solutions work, how to. A buffer solution is a mixture of a weak acid and its. a buffer solution is a solution that resists ph change on dilution or. Titration Buffer Solution.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Buffer Solution a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. Substances which change color with ph. Titration Buffer Solution.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration Titration Buffer Solution Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. Learn how buffer solutions work, how to. a buffer solution is a solution that resists ph change on dilution or addition of acid or base. learn how to use a buret. Titration Buffer Solution.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Titration Buffer Solution A buffer solution is a mixture of a weak acid and its. a buffer solution is a solution that resists ph change on dilution or addition of acid or base. Learn how buffer solutions work, how to. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that. Titration Buffer Solution.
From app.jove.com
Buffers, Buffer Components and Buffer Action Concept Chemistry JoVe Titration Buffer Solution Learn how buffer solutions work, how to. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer. Titration Buffer Solution.
From exportcenter.vniiftri.ru
Titration standards for preparation of buffer solutions рН working Titration Buffer Solution Find out how to determine the. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. a buffer solution is a solution that resists ph change on dilution or addition of acid or base. A buffer solution is a mixture of a. Titration Buffer Solution.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Buffer Solution a buffer solution is a solution that resists ph change on dilution or addition of acid or base. Find out how to determine the. Learn how buffer solutions work, how to. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. A. Titration Buffer Solution.
From www.sliderbase.com
Buffers Presentation Chemistry Titration Buffer Solution A buffer solution is a mixture of a weak acid and its. a buffer solution is a solution that resists ph change on dilution or addition of acid or base. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. a. Titration Buffer Solution.
From www.studocu.com
4 CTEC233 Titrations buffers CTEC 233 CHEMICAL ANALYSIS Titration Buffer Solution A buffer solution is a mixture of a weak acid and its. Find out how to determine the. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. Substances which change color with ph ⇒ they are used to signal the end point. Titration Buffer Solution.
From www.youtube.com
BUFFERS!!! pH meter calibration and intro to titrations YouTube Titration Buffer Solution A buffer solution is a mixture of a weak acid and its. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. Find out how to determine the. a buffer solution is a solution that resists ph change on dilution or addition. Titration Buffer Solution.
From www.researchgate.net
Titration calorimetric traces of a 5 mg/mL SHR2042 and a 200 mM SNAC Titration Buffer Solution learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. Learn how buffer solutions work, how. Titration Buffer Solution.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Titration Buffer Solution a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Find out how to determine the. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is.. Titration Buffer Solution.
From exojbdfvv.blob.core.windows.net
Colorimetric Titration Definition In Chemistry at Maureen Masters blog Titration Buffer Solution a buffer solution is a solution that resists ph change on dilution or addition of acid or base. Substances which change color with ph ⇒ they are used to signal the end point of titrations the ph region in which an indicator changes color is. Learn how buffer solutions work, how to. learn how to use a buret. Titration Buffer Solution.
From mungfali.com
Ph Titration Curve Titration Buffer Solution learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. Find out how to determine the. A buffer solution is a mixture of a weak acid and its. a mixture of a weak acid and its conjugate base (or a mixture of. Titration Buffer Solution.
From www.slideshare.net
Water And Buffers Titration Buffer Solution a buffer solution is a solution that resists ph change on dilution or addition of acid or base. A buffer solution is a mixture of a weak acid and its. a mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution,. Learn how. Titration Buffer Solution.
From dxonsvczs.blob.core.windows.net
Titration Base Concentration at James Cummings blog Titration Buffer Solution Learn how buffer solutions work, how to. Find out how to determine the. A buffer solution is a mixture of a weak acid and its. learn how to use a buret to deliver measured volumes of an acid or a base solution to a flask that contains an unknown solution of. a buffer solution is a solution that. Titration Buffer Solution.